Appointments can feel short. Make sure the conversation with their doctor is a productive one
Use this doctor discussion guide to help you prepare for your child’s visit. That way, you can have a better conversation about starting them on CRYSVITA.
XLH is a lifelong, progressive disease, which means it may continue to get worse over time. Talk to your child’s doctor about CRYSVITA and if it could be right for them.

Discontinue oral phosphate and/or active vitamin D analogs (eg, calcitriol, paricalcitol, doxercalciferol, calcifediol) 1 week prior to initiation of treatment.
If you miss a dose, be sure to receive your next CRYSVITA dose as soon as possible. To avoid missed doses, CRYSVITA may be given up to 3 days before or after the scheduled treatment date.

Discontinue oral phosphate and/or active vitamin D analogs (eg, calcitriol, paricalcitol, doxercalciferol, calcifediol) 1 week prior to initiation of treatment.
If you miss a dose, be sure to receive your next CRYSVITA dose as soon as possible. To avoid missed doses, CRYSVITA may be given up to 3 days before or after the scheduled treatment date.
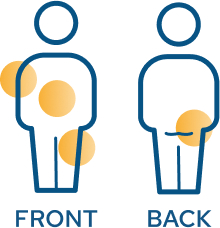
Your child will receive an injection in 1 of 4 places, to be rotated with each injection:
CRYSVITA works to treat XLH as long as your child continues to receive it as prescribed.

Connect with a Kyowa Kirin Cares case manager, who can help you understand your financial options.

Use this doctor discussion guide to help you prepare for your child’s visit. That way, you can have a better conversation about starting them on CRYSVITA.
Leah, age 16,
Living with XLH


Learn more about CRYSVITA, connect with a support community, and receive invites to important educational events.

CRYSVITA is a prescription medicine used to treat adults and children 6 months of age and older with X-linked hypophosphatemia (XLH).
You should not take CRYSVITA if:
What is the most important information you should know about CRYSVITA?
What are the possible side effects of CRYSVITA?
Adverse reactions that were seen in children with XLH are:
Adverse reactions that were seen in adults with XLH are:
Before taking CRYSVITA, tell your doctor about all of your medications (including supplements) and medical conditions, including if you:
While taking CRYSVITA, tell your doctor if you experience:
These are not all the possible side effects of CRYSVITA. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Kyowa Kirin, Inc. at 1-844-768-3544.
For important risk and use information, please see the full Prescribing Information for CRYSVITA.
CRYSVITA is a prescription medicine used to treat adults and children 6 months of age and older with X-linked hypophosphatemia (XLH).
You should not take CRYSVITA if:
What is the most important information you should know about CRYSVITA?
What are the possible side effects of CRYSVITA?
Adverse reactions that were seen in children with XLH are:
Adverse reactions that were seen in adults with XLH are:
Before taking CRYSVITA, tell your doctor about all of your medications (including supplements) and medical conditions, including if you:
While taking CRYSVITA, tell your doctor if you experience:
These are not all the possible side effects of CRYSVITA. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Kyowa Kirin, Inc. at 1-844-768-3544.
For important risk and use information, please see the full Prescribing Information for CRYSVITA.