Increasing and maintaining
phosphorus levels in the blood
Starting on CRYSVITA may
help manage your TIO


Talk to your doctor about CRYSVITA, a treatment proven to help with:
Helping to heal osteomalacia
(weak or softened mature bone)
Talk to your doctor about dosing for CRYSVITA
Your doctor may discuss the following:
- When starting CRYSVITA, the recommended dose is once every 4 weeks for adults and once every 2 weeks for children
- The dosage (the amount of CRYSVITA you take) is based on body weight and will be determined by your doctor. In some cases, this may require more than 1 injection
- After initial treatment, your doctor may have to adjust your dose
Discontinue oral phosphate and/or active vitamin D analogs (eg, calcitriol, paricalcitol, doxercalciferol, calcifediol) 1 week prior to initiation of treatment.
If you miss a dose, be sure to receive your next CRYSVITA dose as soon as possible. To avoid missed doses, CRYSVITA may be given up to 3 days before or after the scheduled treatment date.
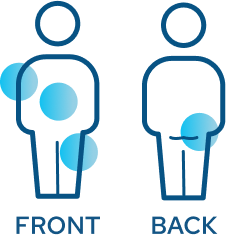
CRYSVITA is given as an injection under the skin by a healthcare provider
You will receive an injection in 1 of 4 places, to be rotated with each injection:
- Upper arm
- Upper thigh
- Buttocks
- Stomach
If the tumor can't be removed, CRYSVITA may help to treat your TIO.

Have questions about access and reimbursement?
Connect with a Kyowa Kirin Cares case manager who can help you understand your financial options.

Stay informed
Learn more about CRYSVITA, connect with a support community, and receive invites to important educational events.
